Neural mechanisms of even the most basic approach-avoidance behavioral decisions are poorly understood, yet understanding how the brain regulates social behaviors is both fundamentally intriguing and also necessary to address various disorders involving social anxiety, depression, and aggression. Understanding the neuroendocrine circuits that regulate affiliative and aversive social behaviors is therefore a primary goal of my research. My current emphasis is on identifying specific populations neurons that produce signaling molecules involved in stress and social behavior signaling (distinct groups of vasotocin, mesotocin, dopamine, norepinephrine, corticotropin-releasing factor, and serotonin cells), as well as their target sites (Fig. 1). Neural activity levels of these cells and of their target sites modulates an individual's social behavior decision making when it comes to performing aggressive or reproductive behaviors. Furthermore, I examine how changes in circulating sex steroid hormones (primarily androgens and estrogens) regulate activity levels within portions of these neural networks.
To conduct this research, I examine lizard model systems, primarily within the genus Anolis. The involved neurotransmitters and brain regions are highly conserved with those in mammals and birds, but the lizard brains are extremely small and relatively simple, thus allowing for whole-brain analyses. Lizards are also not bred for docility and captive housing (like laboratory rodents), and thus exhibit high levels of stereotyped aggression displays and natural fluctuations in steroid hormone levels.
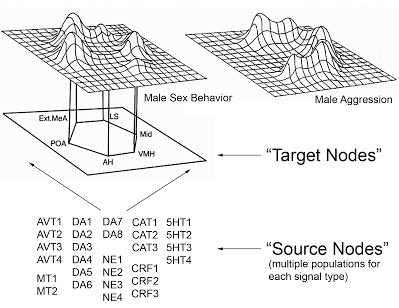
Figure 1 (Click on picture for larger version): A model of the social behavior neural network (adapted from Newman 1999)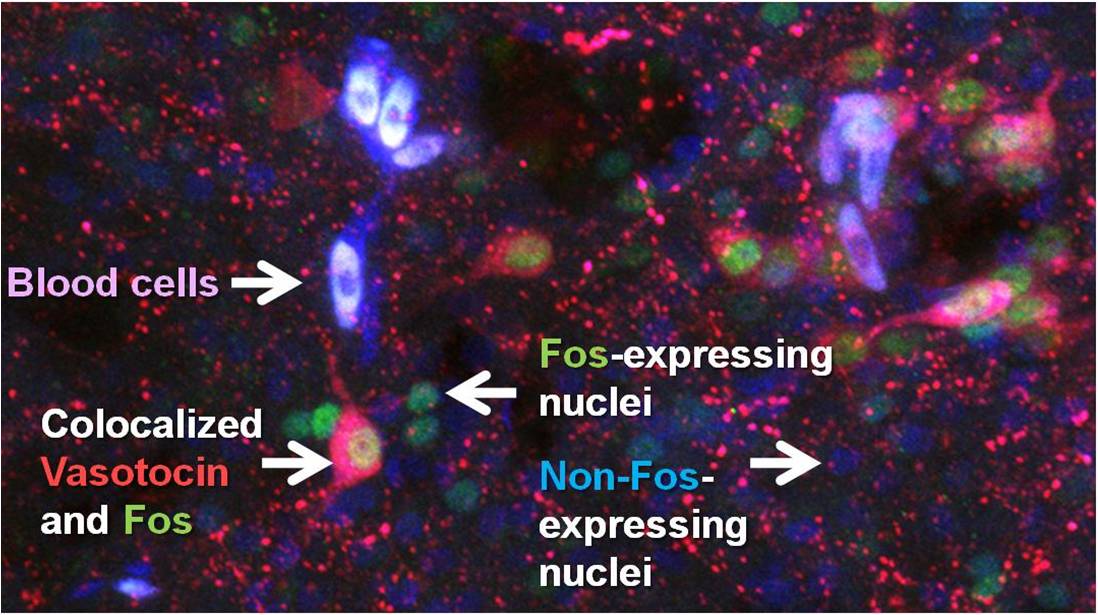
Figure 2: Fluorescent colocalization of the neuropeptide vasotocin (red) with the immediate early gene product Fos (green) allows us to determine which vasotocin neurons were active during a social encounter - in the brown anole (Anolis sagrei).
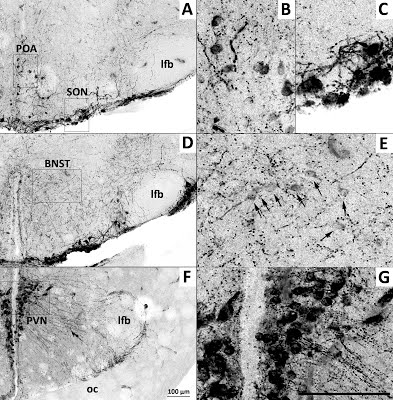
Figure 3: Four distinct arginine vasotocin populations within the brain of the Brown Anole, Anolis sagrei.
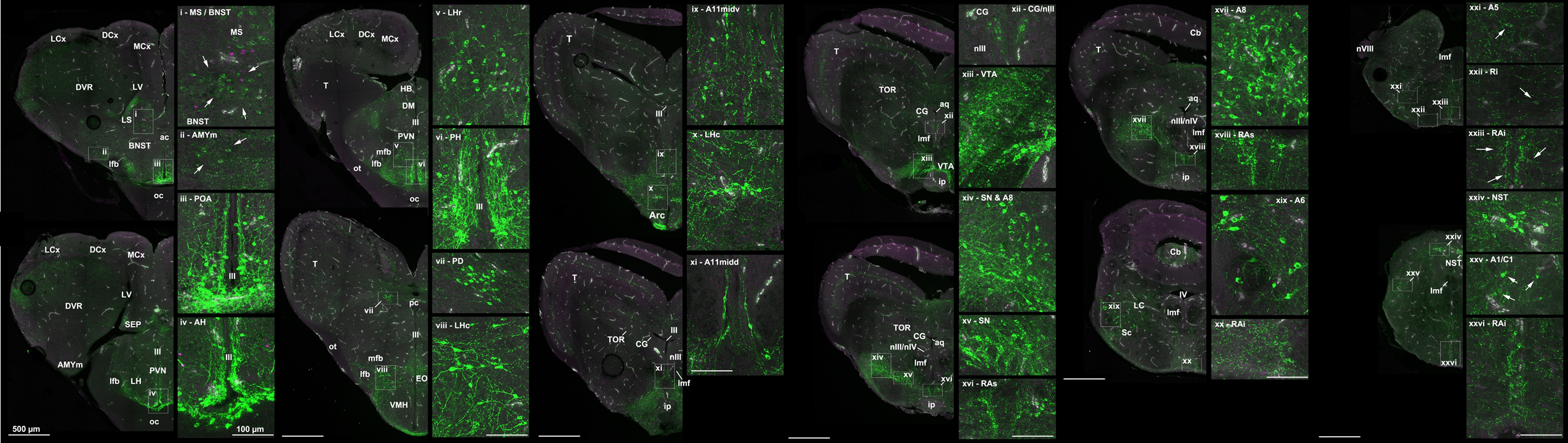
Figure 4: More than twenty distinct catecholamine populations within the brain of the Brown Anole, Anolis sagrei.